The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public notification about the recall of Johnson & Johnson’s Benylin Paediatric Syrup.
This action comes in the light of recent laboratory findings indicating toxicity in the product.
Laboratory analysis conducted on the affected lot revealed an alarming presence of Diethylene Glycol, prompting concerns over its safety.
Diethylene Glycol, a known toxic substance, can have severe consequences upon ingestion, including abdominal pain, vomiting, and acute kidney injury, which can be fatal.
Benylin Paediatric Syrup is commonly used for alleviating cough and its associated symptoms, as well as treating hay fever and other allergic conditions in children aged 2 to 12 years.
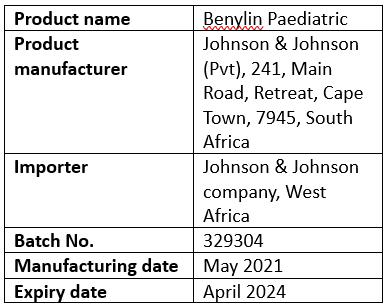
Product details
What you should know
NAFDAC emphasises the importance of caution and vigilance among importers, distributors, retailers, and consumers throughout the supply chain to prevent the importation, distribution, sale, and use of substandard or contaminated regulated products.
- It is crucial to procure medical products only from authorised and licensed suppliers, ensuring their authenticity and physical condition are thoroughly checked.
- Individuals in possession of the affected product are urged to cease its sale or use immediately and submit remaining stock to the nearest NAFDAC office.
Furthermore, any adverse reactions observed following the use of this product should be promptly reported to qualified healthcare professionals for immediate medical attention.
Healthcare professionals and consumers are encouraged to report suspicions of substandard or falsified medicines to the nearest NAFDAC office or through various reporting channels provided by NAFDAC.
The Agency has directed the Marketing Authorisation Holder, Johnson and Johnson Company West Africa, to initiate the recall process for the affected batch, with notices to be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).


