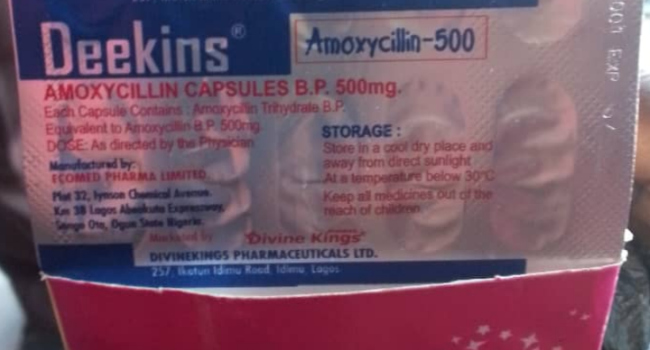
The National Agency for Food and Drug Administration and Control (NAFDAC) has announced the recall of a specific batch of Deekins Amoxycillin 500mg capsules.
In a statement shared on X (formerly Twitter) on Wednesday, NAFDAC identified the affected batch as lot number 4C639001, manufactured by Eco-med Pharma Ltd and distributed by DevineKings Pharmaceutical Ltd.
The recall was initiated following reports of severe adverse drug reactions linked to this batch.
According to Eco-med Pharma Ltd, a hospital reported three cases of serious reactions in patients who were given capsules from the recalled batch.
The statement said, “NAFDAC is notifying the public of the recall of one batch of Deekins Amoxycillin 500mg Capsules, manufactured by Eco-med Pharma Ltd and marketed by DevineKings Pharmaceutical Ltd, with lot number 4C639001.
“This batch is recalled following reports of serious adverse drug reactions.
“According to Eco-med Pharma Ltd, reports of serious adverse drug reactions were received from a hospital that reported three cases of serious adverse drug reactions from patients administered with the batch of Deekins Amoxycillin 500mg capsule.
“Amoxicillin is a penicillin antibiotic indicated for treating bacterial infections such as tonsillitis, bronchitis, sinusitis, pneumonia, and bacterial infections of the ear, nose, throat, skin, or urinary tract.
NAFDAC also advised healthcare professionals and consumers to immediately stop using the affected batch and to report any suspicious cases of substandard or falsified medicines to the nearest NAFDAC office.
“Healthcare professionals and consumers are advised to report any suspicion of substandard and falsified medicines to the nearest NAFDAC office, call 0800-162-332,2 or send an email to [email protected]