The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public statement regarding the European Union’s (EU) ban on the sale of Dex Luxury Bar Soap (No 6 Mystic Flower).
The EU has forbidden the sale of this product due to noncompliance with the Cosmetic Products Regulation, as it apparently contains Butyphenyl Methylpropional (BMHCA), a chemical considered hazardous to the reproductive system.
This ingredient poses risks to the health of unborn children and may also cause skin sensitization, prompting regulatory and public authorities in the EU to impose a ban on its marketing.
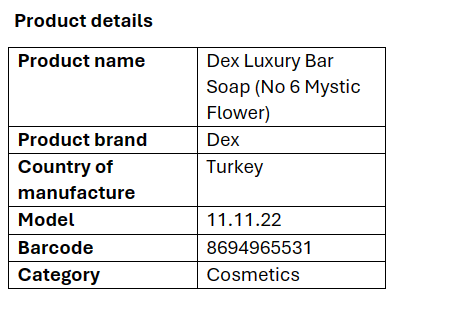

Although Dex Luxury Bar Soap (No 6 mystic flower) is not listed in the NAFDAC database, importers, distributors, retailers, and consumers are advised to exercise caution and vigilance within the supply chain to prevent the importation, distribution, sale, and use of the product in question.
Thorough checks on the product’s authenticity and physical condition are recommended.
Individuals in possession of the banned product are urged to discontinue its sale or use and submit their stock to the nearest NAFDAC office.
Healthcare professionals and consumers are encouraged to report any suspicions of adverse reactions or the presence of substandard and falsified regulated products to the nearest NAFDAC office or through designated communication channels provided by the agency.
Similarly, healthcare professionals and patients are advised to report any adverse events or side effects associated with the use of regulated products to NAFDAC, utilizing available reporting platforms on the agency’s website or through dedicated e-reporting applications downloadable on Android and IOS devices.



